READSTOWN - The Red Cross began a new initiative in Wisconsin on June 15 to offer free COVID-19 antibody testing to individuals who volunteer to donate blood. The test will tell a person whether or not they have had the virus in the past, but is not a test to show whether or not they currently have the virus.
“We have entered into an initiative with the U.S. Food and Drug Administration (FDA) to provide antibodies testing to blood donors,” Red Cross spokeswoman Laura McGuire said. “We hope to continue offering the test at least through the end of the summer.”
McGuire explained that Red Cross results would be shared with state public health departments to assist them in understanding the prevalence, or how widespread the virus is, in their states.
“The data we share will observe HIPAA healthcare privacy laws, and no individual donors information will be shared,” McGuire said. “What will be shared will just be the total positive and negative numbers coming out of a blood drive.”
On Saturday, July 11, 41 individuals gave blood in Readstown, and antibody testing was provided at that blood drive. Red Cross will not notify donors of their results, but donors can find their results by logging into their account at www.redcrossblood.org.
Results will become available within 7 to 10 business days after the testing event. An individual can also download the Red Cross app onto their smartphone, and receive the results directly through that app. For information about how to download the app, go to https://www.redcross.org/get-help/how-to-prepare-for-emergencies/mobile-apps.html.
Upcoming drives
Those interested in donat-ing blood and having it test-ed for COVID-19 antibodies can consider signing up for one of these upcoming blood drives.
• July 28: Church of Christ, 825 Nelson Pkwy, Viroqua, 12-5:30 p.m.
• July 28: United Method-ist Church, 625 Dousman St. Prairie du Chien, 2-5 p.m.
• July 30: Memorial Building, 860 Lincoln Ave., Fennimore, 11:30 a.m.-5:30 p.m.
• July 31: Prairie du Chien Library, 125 S. Wacouta Ave., Prairie du Chien, 11:30 a.m.-5:30 p.m.
• August 6: Wisconsin Secure Program Facility, 1101 Morrison Dr., Boscobel, 10 a.m. – 3 p.m.
• August 21: Gays Mills Community Building, 16381 State Highway 131, Gays Mills, 1-5 p.m.
Antibodies tests
According to the FDA website, ‘serology’ or ‘antibody’ tests detect the presence of antibodies in the blood when the body is responding to a specific infection, like COVID-19. In other words, the tests detect the body's immune response to the infection caused by the virus rather than detecting the virus itself.
In the early days of an infection, when the body's immune response is still building, antibodies may not be detected. This limits the test's effectiveness for diagnosing COVID-19.
Serology tests could play a role in the fight against COVID-19 by helping healthcare professionals identify individuals who may have developed an immune response to SARS-CoV-2. In addition, these test results can aid in deter-mining who may donate a part of their blood called convalescent plasma, which may serve as a possible treatment for those who are seriously ill from COVID-19.
The performance of these tests is described by their "sensitivity," or their ability to identify those with anti-bodies to SARS-CoV-2 (true positive rate), and their "specificity," or their ability to identify those without antibodies to SARS-CoV-2 (true negative rate).
A test's sensitivity can be estimated by determining whether or not it is able to detect antibodies in blood samples from patients who have been confirmed to have COVID-19 with a nucleic acid amplification test, or NAAT. In some validation studies of these tests, like the one FDA is conducting in partnership with NIH, CDC, and BARDA, the samples used, in addition to coming from patients confirmed to have COVID-19 by a NAAT, may also be confirmed to have antibodies present using other serology tests.
A test's specificity can be estimated by testing large numbers of samples collect-ed and frozen before SARS-CoV-2 is known to have circulated to demonstrate that the test does not pro-duce positive results in re-sponse to the presence of other causes of a respiratory infection, such as other coronaviruses.
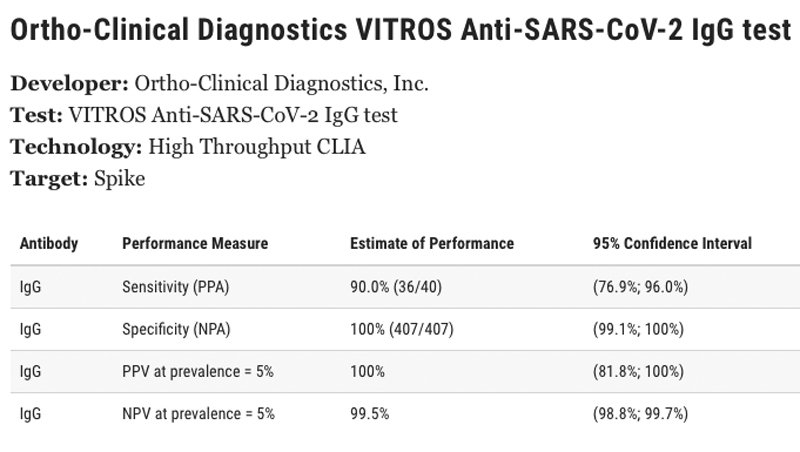
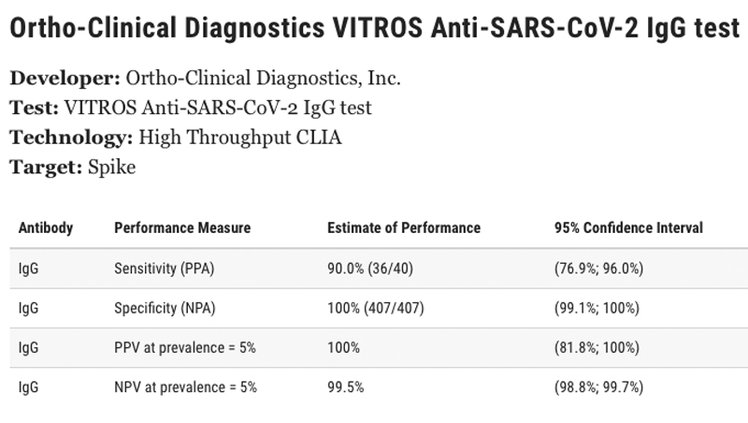
Which test was it?
The COVID-19 antibody test currently being offered free of charge to Red Cross blood drive donors is the VITROS Immunodiagnostic Products Anti-SARS-CoV-2 IgG Reagent Pack - Ortho-Clinical Diagnostics, Inc.
The sensitivity of the test is 90 percent (36/40). The specificity of the test is shown on the FDA website as 100 percent. The positive predictive value (PPV) of the test at prevalence is 100 percent. The negative predictive value of the test at prevalence (NPV) is 99.5 percent.
The test is one on a list of COVID-19 antibodies tests listed on the FDA website as approved for Emergency Use Authorization (EUA) due to the urgency of the health care crisis. To see the list and for more information about antibodies testing, go to https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance.
The EUA for this test is supported by the Secretary of Health and Human Service’s declaration that circumstances exist to justify the emergency use of in vitro diagnostics for the detection and/or diagnosis of the virus that causes COVID-19.
Test results meaning
If you have a positive test result, it is likely that you have or previously had COVID-19 and that you have developed an antibody response to the virus. Your healthcare provider will work with you to determine how best to care for you based on the test results along with other factors of your medical history, including any previous symptoms, possible exposure to COVID-19, and the location of places you have recently traveled.
A negative test result means that the antibodies to the virus that causes COVID-19 were not found in your sample. However, it is possible for this test to give a negative result that is incorrect (false negative) in some people with COVID-19 infection. A negative result may occur if you are tested early in your illness and your body hasn’t had time to produce antibodies to infection.
State initiative
The Wisconsin Department of Health Services (DHS) is partnering with the University of Wisconsin-Madison’s Survey of the Health of Wisconsin (SHOW) and Wisconsin State Laboratory of Hygiene (WSLH), the University of Wisconsin-Milwaukee (UWM), and the Wisconsin Department of Natural Resources to conduct two population health studies that will examine the presence of COVID-19 in Wisconsin.
Together, these studies will provide researchers and public health experts with a better understanding of where COVID-19 has been and help identify communities that may be at risk for future outbreaks. This research will be a valuable tool for informing future public health practices while maximizing containment efforts.
“One of the most challenging things about COVID-19 is that as a new virus, there is still so much to learn”, said DHS Secretary-designee Andrea Palm. “That is why this type of research is essential to our statewide efforts and until there is a vaccine, we must do everything we can to protect our communities and ensure that we are safely reopening the state.”
The first study, Past Antibody COVID-19 Community Survey (PACCS) is led by SHOW and will determine the prevalence of COVID-19 antibodies throughout the state. Antibodies indicate if a person has been infected with COVID-19 in the past, even if they did not experience symptoms.
Antibody testing helps in understanding how many people were infected with COVID-19; it does not provide information regarding the current amount of positive cases, and is not an alternative to diagnostic testing. Study participants will receive antibody testing quarterly over the course of the next year.
“The Survey of the Health of Wisconsin (SHOW) will recruit individuals who have participated in SHOW in the past. Past participants are from ten randomly selected counties and the City of Milwaukee. The participants represent a population of residents from across the entire state,” said Kristen Malecki, Director and Principal investigator for the SHOW program, and associate professor in the Department of Population Health Sciences at the UW School of Medicine and Public Health.
“Our past experience surveying the health of this state, existing research infrastructure, and community partnerships should allow us to aid in the containment and tracking effort of the COVID-19 pandemic."
WSLH is collaborating with UWM and the DNR for the second study, Wastewater Surveillance of SARS-CoV-2 in Wisconsin. This study will test samples from wastewater treatment facilities (WWTF) in both populated areas and rural portions of the state in order to determine the current concentration levels of virus genetic material found in the sewage.
If the virus is detected or virus quantities are increasing, public health officials can proactively adopt measures to minimize transmission of the virus and prepare for a surge. Conversely, wastewater samples may be able to detect areas with low levels of infection.
Surveillance of wastewater will provide public health officials with the opportunity to identify the magnitude of COVID-19 transmission, circulation within a community, and potentially, early warning detection of outbreaks.
Results should begin coming in later this summer, as the WSLH begins routine sampling of wastewater. The study will run for a year, through June of 2021.
“Routinely monitoring COVID-19 in wastewater is an effective method to assess community-wide presence and levels of the virus,” said Dr. Jonathan Meiman, Chief Medical Officer for the DHS Bureau of Environmental and Occupational Health and Occupational Disease Epidemiologist. “The results will help communities with higher concentrations of COVID-19 prepare for a potential surge in cases. This approach is not designed to replace the existing public health surveillance, but will help supplement the current practices and mitigation efforts.”
These two studies, along with Wisconsin’s current wide-scale testing infrastructure, will help communities reduce the risk of COVID-19.